![Products Products]() proprietary and novel site-specific single and dual-payload glycan conjugation platform
proprietary and novel site-specific single and dual-payload glycan conjugation platform
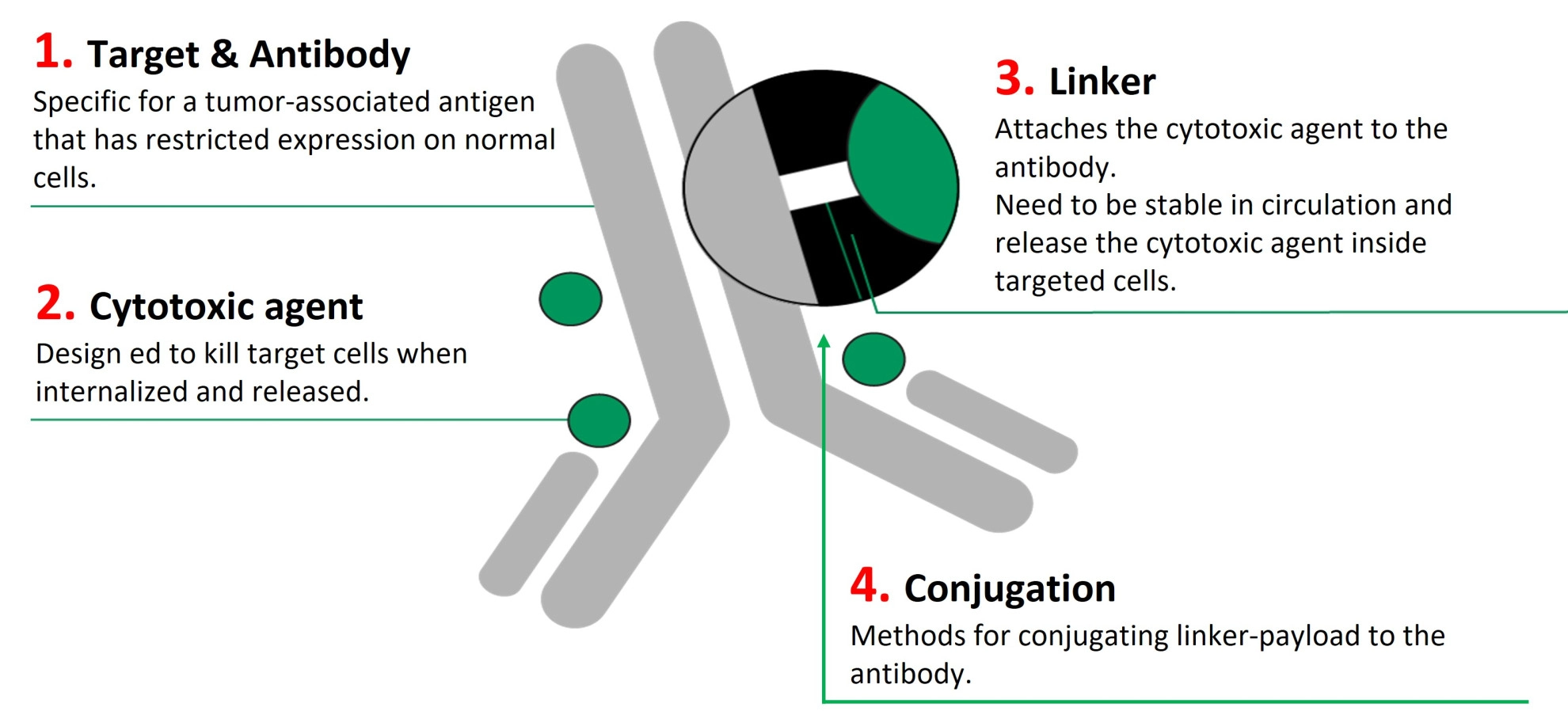
About antibody-drug conjugates (ADC)
Antibody-drug conjugates (ADCs) are a class of cancer therapeutics, in which the drugs are attached to a tumor-targeting antibody through specific linkers and conjugation methods.
References
- Carter PJ et al. Cancer J. 2008;14(3):154-169.
- Senter PD. Curr Opin Chem Biol. 2009;13(3):235-244. 3. Polson AG et al. Cancer Res. 2009;69(6):2358
Unique Conjugation Technology
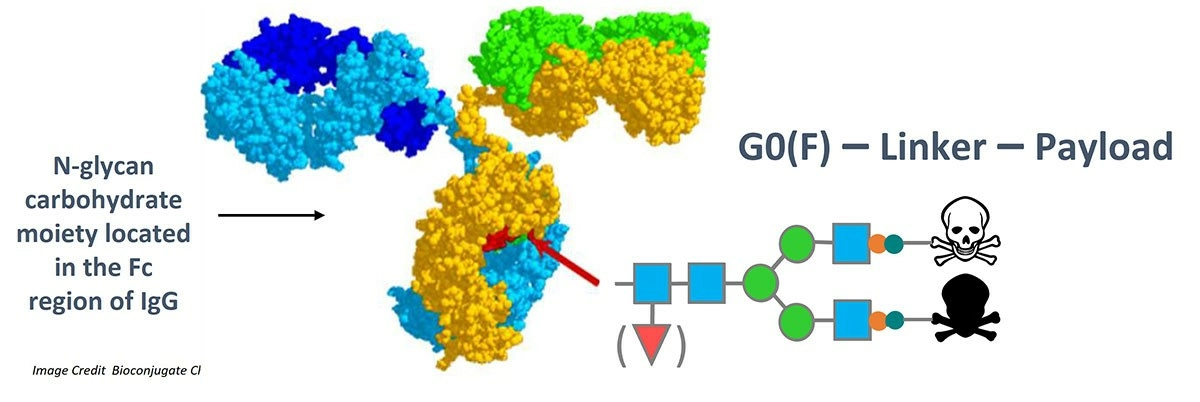
 HoneyBear Biosciences' proprietary conjugation belongs to the new generation of site-specific conjugation technology. The core technology utilizes glycan conjugation.
HoneyBear Biosciences' proprietary conjugation belongs to the new generation of site-specific conjugation technology. The core technology utilizes glycan conjugation.
| Aspect | Advantage |
|---|---|
| Precise conjugation site and conjugation ratio | Improved efficacy, stability and safety |
| No antibody sequence mutation needed | Significant savings in time and cost |
| Can conjugate two different payloads on the same antibody | Wider application, increased efficacy and decreased drug resistance |
| ADC products are more homogeneous | Reduced manufacturing costs and risks; increased speed |
| High yield enzymatic conjugation with rapid purification | Time and cost savings |
Facile dual-payload synthesis

Combination of Different MOA Payloads
Enhanced efficacy; decreased resistance; immune stimulation
Different MOA Payloads
- Tubulin inhibitors; DNA damaging agents; topo-I inhibitors
- Immune modulators
- Radioisotopes
- Protein-degraders
CoNectar®: proprietary and novel site-specific single and dual-payload glycan conjugation platform
Leveraging HoneyBear's Technologies to Revolutionize Next Generation ADCs
1. Facile dual payload synthesis addresses high barriers to entry
2. Dual payload concepts address multiple medical challenges faced by single payload ADCs
- Low response rates
- Short duration of response
- Resistance
3. Dual payload technologies open the door to attacking tumors from multiple fronts
- Different yet complementary Mechanisms of Action
- Additive or synergistic effects
- Induction of endogenous anti-cancer immune responses to achieve long term remissions
4. First-in-class and Best-in-class agents to command enormous market potential
HoneyBear's patented CoNectar® Platform

US11085062B2

JP06888764B2

CA3048452C

AU201717388556B2
TWI673363B

KR2294517B1
Patent family: Process For Preparing Glycoprotein-Drug Conjugates
- Anticipated expiration: 2037-12-29
- Priority Date: US Provisional 62/440075, 29 Dec. 2016
- WO2018126092A1, EP3568161A4, CN110121365A (OA)